Currently, there are several methods available for removing MPs, such as membrane filtration, biodegradation, adsorption, photocatalytic degradation, coagulation, and electrocoagulation. These technologies are installed in combination or individually for the efficient and effective removal of MPs from aquatic environments. However, the efficiency of each technique varies according to the MPs’ dimensions, form, and chemical composition. Furthermore, some of these technologies are either less efficient or consume more energy. As a result, environmental practitioners are now attempting to develop more effective and efficient MP removal technologies.
Coagulation is a promising technique employed in wastewater treatment plants (WWTPs) to remove suspended particles from the effluent. Recently, it has attracted a lot of scientific interest as a viable method for removing MPs from aquatic environments due to its simplicity, reduced operational costs, low carbon footprint, and eco-friendly features. Furthermore, the coagulation technique is more viable for large-scale wastewater treatment applications with less time required for purification as compared to other conventional methods.
Adding synthetic or natural polymers to water during flocculation helps settle unstable particles of MPs by fusing with a flocculant to form bigger microflocs. Various inorganic flocculants can remove MP particles by compressing the electric double layer (EDL). However, the low removal efficiency of MPs was reported with common coagulants due to variations in size, density, and shape, as well as inadequate coagulant hydrolysis. According to Ma et al. (2019), the removal efficiencies of FeCl3·6H2O and AlCl3·6H2O coagulants were below 12.7% and 36.9%, respectively, for polyethylene (PE) particles with a size range of 0.5-5 mm. Recent research by Zhang et al. found that Al2(SO4)3 had a removal efficiency of less than 2% for MPs (PE and PS) (180 nm - 125 μm).
The floc adsorption and sedimentation capacities, floc volume, and floc density, as well as the removal of MPs via coagulation, can all be enhanced using coagulant aids. The removal efficiency of MPs (PE) (0.5 mm) was increased from 25% to 61% when cationic polyacrylamide (PAM) was added to AlCl3·6H2O. As a result, various experimental conditions must be used to improve the removal efficiency of MPs during coagulation processes.
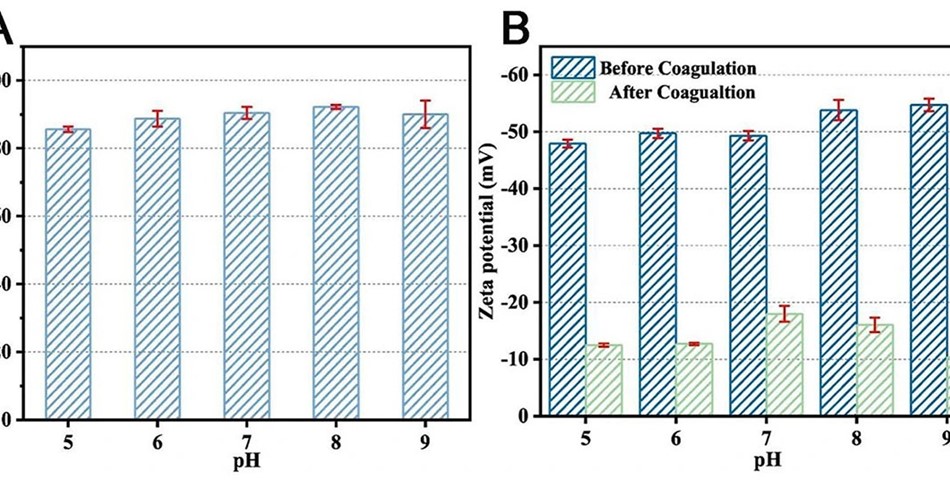
Coagulation by charge neutralization involves the addition of highly charged cations that neutralize the surface charge on colloidal particles, leading to their aggregation and removal from the water. In this process, the positively charged cations such as Al3+ or Fe3+ are attracted to the negatively charged colloid particles and adsorb onto their surface, which neutralizes their surface charge and eliminates repulsive forces between them. As a result, strong van der Waals forces are generated, which cause the aggregation of particles into larger flocs. These flocs have the ability to capture and remove colloidal particles. However, hydrolysis may occur, leading to the formation of insoluble hydroxides, depending on the concentration of the coagulant and the pH of the solution. These hydroxides can contribute to the development of even bigger aggregates, which can then be removed through sweep coagulation. To fathom the coagulation mechanisms, it is necessary to investigate the basic principles that govern the interaction between MPs and coagulants or coagulant aids.
Recent studies found that the main factor for the flocculation of aluminum oxide particles and surface-functionalized 2-acrylamido-2-methylpropionic acid polymer nanoparticles was electrostatic interaction. In addition, Fourier transform infrared spectroscopy (FTIR) research showed that MPs’ surfaces had formed new Al-O and Fe-O bonds, which contributed to their enhanced settling. It is necessary to conduct additional research on the chemical interactions that take place between MPs and flocs, as well as research into the prevailing factors (Brownian motion or buoyancy force) that determine the settling of MPs.
Despite the enormous studies conducted on coagulation technology, there are few literature reviews that comprehensively summarize removal mechanisms along with the gaps and progress on the coagulation technology for eliminating MPs from aqueous solution. Considering this, the current review has been designed with the aim of (i) assessing the effectiveness of the coagulation technique for MP removal from water and wastewater; (ii) investigating the effect of experimental factors such as coagulant type, characteristics of MPs, and operating conditions on the removal efficiency of MPs via coagulation; (iii) conducting a systematic analysis of the mechanism by which removal of MPs is accomplished by coagulation; (iv) exploring the potential of bio-based and eco-friendly coagulants for the removal of MPs; and (v) highlighting the opportunities and challenges of coagulation for efficient MP removal.
The findings of the current study can be used to increase the removal efficiency of MPs from water via coagulation in WWTPs by providing insight into the mechanism by which particle size and water chemical conditions affect the removal of nanosized plastic particles.