Discharged in significant quantities by textile, cosmetic, ink, paper, and other manufacturers, dyes pose a high toxicity risk and can introduce potential carcinogens into wastewater. This presents a significant challenge in wastewater treatment. However, researchers at Drexel University's College of Engineering may have discovered a solution utilizing a minute nanofilament.
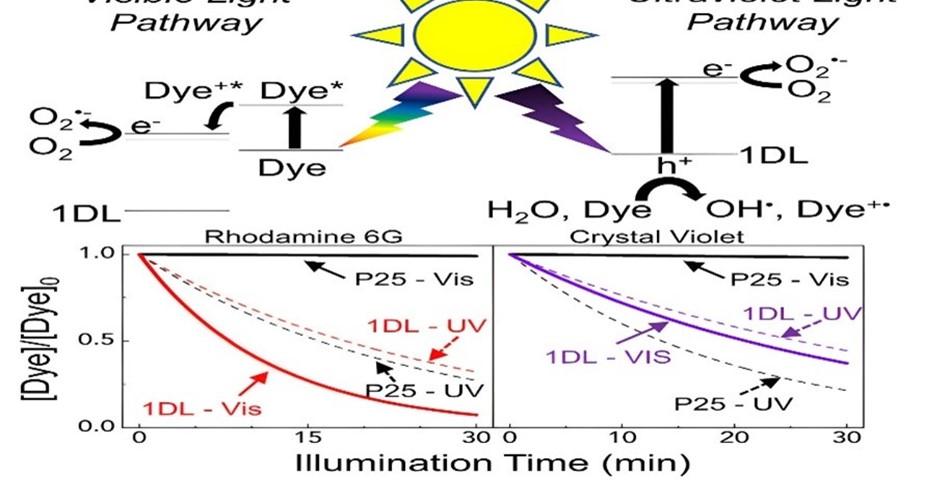
Lead by Michel Barsoum, Ph.D., a Distinguished University professor in the College of Engineering, the research team, which included researchers from Drexel's College of Arts and Sciences, identified a one-dimensional titanium oxide photocatalyst material with a lepidocrocite structure. This material has the capability to break down two common dye pollutants, rhodamine 6G and crystal violet, under visible light. In just 30 minutes, the material reduced the concentrations of these dyes in the water by 90% and 64%, respectively, when the catalyst-to-dye ratio was 1 to 1.
This discovery is particularly promising as it addresses a long-standing issue in water treatment. Integrating this titanium-oxide photocatalyst into existing processes could potentially enhance its effectiveness in removing these chemicals and reduce the energy required to do so.
The process begins with adsorption, where the dye adheres to the nanofilament's surface, and when exposed to light, it undergoes photocatalysis. The dye sensitizes the nanofilaments to visible light, accelerating degradation and causing the dye to break down into harmless byproducts like carbon dioxide and water.
The study, recently published in the journal Matter, revealed that the key to the dye degradation and self-sensitization process lies in the material's ability to generate electron holes and various radicals such as hydroxyl, superoxide, singlet oxygen, as well as electron "holes."
Rhodamine 6G and crystal violet are commonly found dye pollutants in wastewater. Unlike sewage, effluent, which means "something that flows out," is challenging to remove from water because it remains suspended. These dyes are water-soluble, and any excess is discharged as effluent.
Wastewater is a significant environmental concern worldwide, affecting human health, aquatic life, and plants in the long term. Globally, households and industries generate nearly 380 billion cubic tons of wastewater each year, and only 24% of it is treated sufficiently due to various challenges, including high energy consumption, residual chemicals, staffing at treatment centers, and inadequate processing of complex and persistent contaminants, such as dyes.
Traditional wastewater treatment methods like sedimentation, biological oxidation, and chemical-physical treatment are ineffective at removing dyes due to their complex molecular structure and water-soluble nature. While adsorption with materials like clay, activated carbon, and iron oxide has been used, these materials merely separate the dye from the water without breaking it down.
Photocatalysts, previously considered the solution, often require UV light treatment, which is energy intensive. The novel aspect of nanofilament lies in its self-sensitization behavior, making it highly responsive to visible light.
Using visible light, which is visible to the human eye, can significantly reduce the costs associated with treatment and energy consumption while effectively removing dyes from wastewater and eliminating toxic effluents. This also opens opportunities for other applications in fields like solar cells and optical devices.
The outcome is cleaner water without the need for additional toxins or extra energy consumption. To conduct the study, the team used various techniques such as x-ray diffraction, electron microscopy, and ultraviolent-visible spectroscopy to characterize the nanomaterial and monitor dye degradation.
One of the most significant findings of the study is that the nanofilament is sensitized by the dye, creating a mutually beneficial relationship that results in cleaner and less toxic water. Essentially, the dye catalyzes its own destruction.
Furthermore, while this study demonstrates the nanofilament's potential to improve water treatment, it also suggests that the material can be sensitized, opening doors to applications in solar cells and optical devices. In an earlier study, the same nanofilament was found to harness sunlight for hydrogen separation, highlighting its potential in green fuel generation.
Michel Barsoum expressed the excitement of uncovering the possibilities of this material, and as its behavior becomes better understood, it may have applications in various technologies needed for a more sustainable future.
Discover additional information – click here to learn more!